MEDIA _
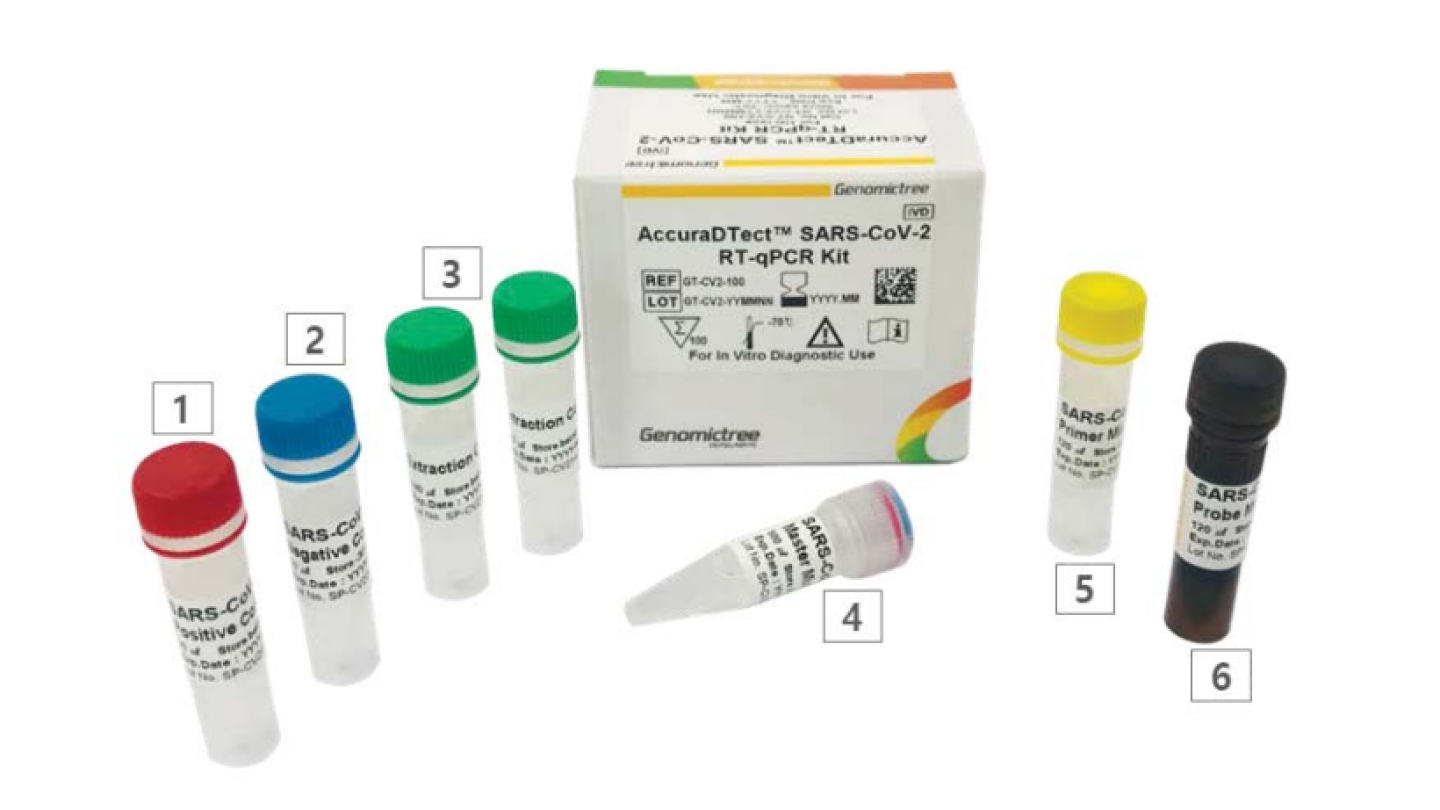
Daewoong Pharmaceutical Company Indonesia (DPCI) Acquires a License 10000 to Sell COVID-19 Test Kit in Indonesia
▲ Daewoong Pharmaceutical Company Indonesia announced on 22th that it acquired a license to sell ‘AccuraDtect’ SARS-COV-2 RT-qPCR Kit, a molecular test kit for COVID-19 in Indonesia. – Plan to supply ‘AccuraDtect,’ the COVID-19 test kit with CE-IVD certification acquired from Europe to Indonesia– Enable to produce results within 4 hours after sample collection…Expecting to strengthen responsiveness to COVID-19 Daewoong Pharmaceutical Company Indonesia (hereinafter referred to as DPCI) (CEO Sengho Jeon) that is developing COVID-19 treatment announced on 22th that it acquired a license to sell ‘AccuraDtect’ SARS-COV-2 RT-qPCR Kit, a molecular test kit for COVID-19 in Indonesia. “As the…

Joint Ventures build competitiveness in the industry… Turning COVID-19 Crisis into an Opportunity with Localization Strategy
▲.Daewoong Infion, a joint venture between the Korean pharmaceutical company, Daewoong Pharmaceutical, and Indonesian pharmaceutical…

Bio Regenerative Industry Issue Keyword, ‘Stem Cells’ – From COVID-19 Treatments to Bone Regeneration
▲ Daewoong Infion, a joint venture of South Korean pharmaceutical company Daewoong Group in Indonesia,…